AR101 – Enzastaurin
About AR101 (enzastaurin)
Enzastaurin Clinical Development

Prevention of Rupture with Enzastaurin in Vascular Ehlers-Danlos Syndrome
Objective: To evaluate the efficacy of enzastaurin compared to placebo in preventing arterial events leading to intervention in patients with Vascular Ehlers-Danlos Syndrome (VEDS) confirmed with COL3A1 gene mutations.
VEDS/COL3A1 Overview
Upcoming Clinical Trial
Aytu BioPharma, Inc. will be sponsoring an upcoming clinical trial to evaluate the effectiveness of AR101 (enzastaurin) in preventing cardiac or arterial events in patients with Vascular Ehlers-Danlos Syndrome (VEDS) confirmed with COL3A1 gene mutations, compared to placebo.
About Vascular Ehlers-Danlos Syndrome (VEDS)
Vascular Ehlers-Danlos Syndrome (VEDS) is an inherited connective tissue disorder, typically caused by a mutation in the COL3A1 gene. This mutation leads to defects in type III procollagen, a major protein in vessel walls and hollow organs. Patients with this diagnosis are at significant risk for serious vascular events like dissections, pseudoaneurysms, and ruptures throughout the vasculature.
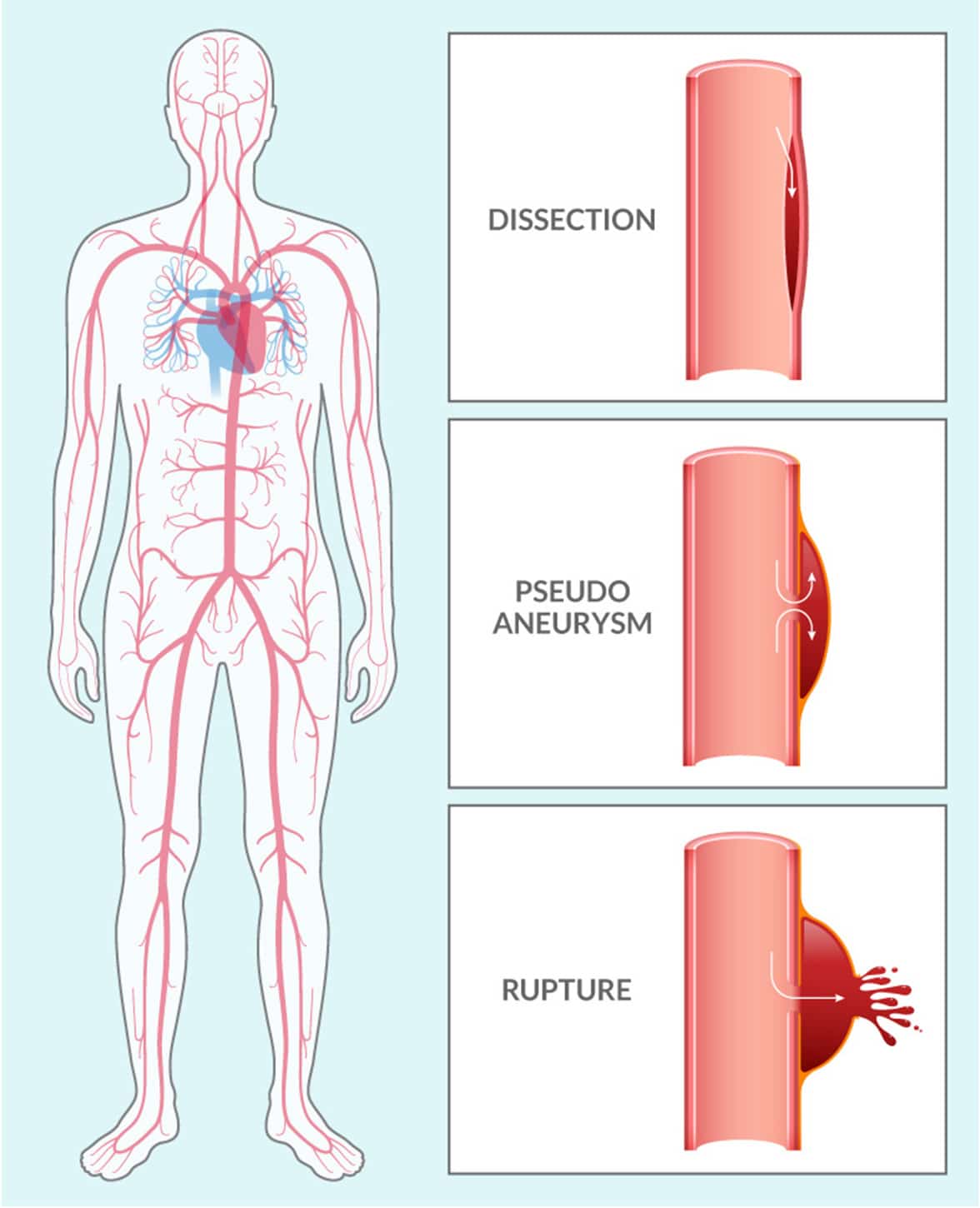
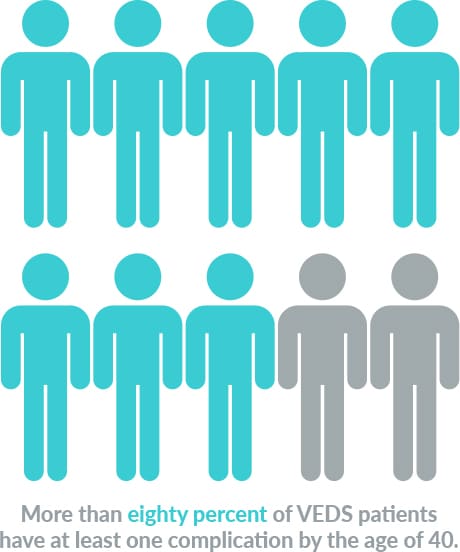
VEDS affects about 1 in 50,000 people worldwide. Nearly 50% of patients with this devastating condition die before the age of 50 years old. Currently, there are no FDA-approved therapies, and after diagnosis, the current standard of care is “watchful waiting.”

Scientific Advisory Board
Hal Dietz, M.D.
Chairman of the Scientific Advisory Board
Bio ![]()
Xavier Jeunemaitre, M.D., Ph.D.
Bio ![]()
Shaine A. Morris, M.D., M.P.H.
Bio ![]()
Peter Byers, M.D.
Bio ![]()
Living with VEDS
Annie
Bridgette
Katie
The Tays Family
Aytu BioPharma is a proud sponsor of these VEDS Advocacy Groups
References
1. https://www.clinicaltrials.gov/ct2/show/NCT00332202. 2. Bowen CJ et al. Targetable cellular signaling events mediate vascular pathology in vascular Ehlers-Danlos Syndrome. J Clin Invest. 2020 Feb 3;130(2):686-698.
N01161 06/22
Expanded Access Policy
Expanded access, also called compassionate use, enables patients with serious or immediately life-threatening diseases who do not meet the enrollment criteria for clinical trials in progress to gain access to investigational treatments. At this time, Aytu BioPharma does not offer an expanded access program and does not accept expanded access requests. We believe that investigational drugs should be studied in patients as part of clinical trials designed to produce data on safety and efficacy that may be used to support approval of the product, thereby leading to its broader availability for patients in need of treatment. Aytu strongly encourages patients to speak with their treating physicians and when possible to participate in clinical trials. Aytu is aware that in rare cases patients with serious life-threatening diseases are unable to participate in clinical trials and may have exhausted all available therapies. In these rare cases, Aytu may consider providing an investigational product outside of a clinical trial. However, we currently do not have an expanded access program that allows patients to have access to our investigational products prior to FDA approval. As authorized by the 21st Century Cures Act, Aytu may revise this expanded access policy at any time. Additionally, the posting of this policy by Aytu shall not serve as a guarantee of access to any specific investigational drug by any individual patient. In the event Aytu decides to consider expanded access, we will evaluate and respond to each request that it receives on a case-by-case basis. Reference information about our investigational drugs and ongoing clinical trials can be found on https://www.aytubio.com and https://clinicaltrials.gov. If you have additional questions, please speak with your physician, or contact us at ExpandedAccess@aytubio.com.





